Data-Driven Manufacturing Operations
Fostering a culture of data-driven decision-making in pharmaceutical manufacturing.

A vision for modern pharmaceutical production — and how to operationalize it.
Pharmaceutical manufacturing has made extraordinary progress in automating the physical steps of drug production. Filling lines, inspection systems, bioreactors, and utilities are increasingly digitalized, controlled, and instrumented.
Yet the industry still faces a fundamental constraint: manufacturing decisions are not as data-driven as the equipment itself.
Much of the cognitive work — planning, troubleshooting, reporting, documentation, investigation, and optimization — remains manual, fragmented across systems, or dependent on local expertise. The result is slow decision-loops, operational friction, and limited ability to scale knowledge.
This page outlines a practical vision for transitioning to data-driven manufacturing operations, focused on biopharmaceutical (drug product) production, i.e., fill-finish (Fig. 1). It starts with the high-level “why,” then moves toward actionable strategies for digital/AI leaders ready to operationalize this transformation.
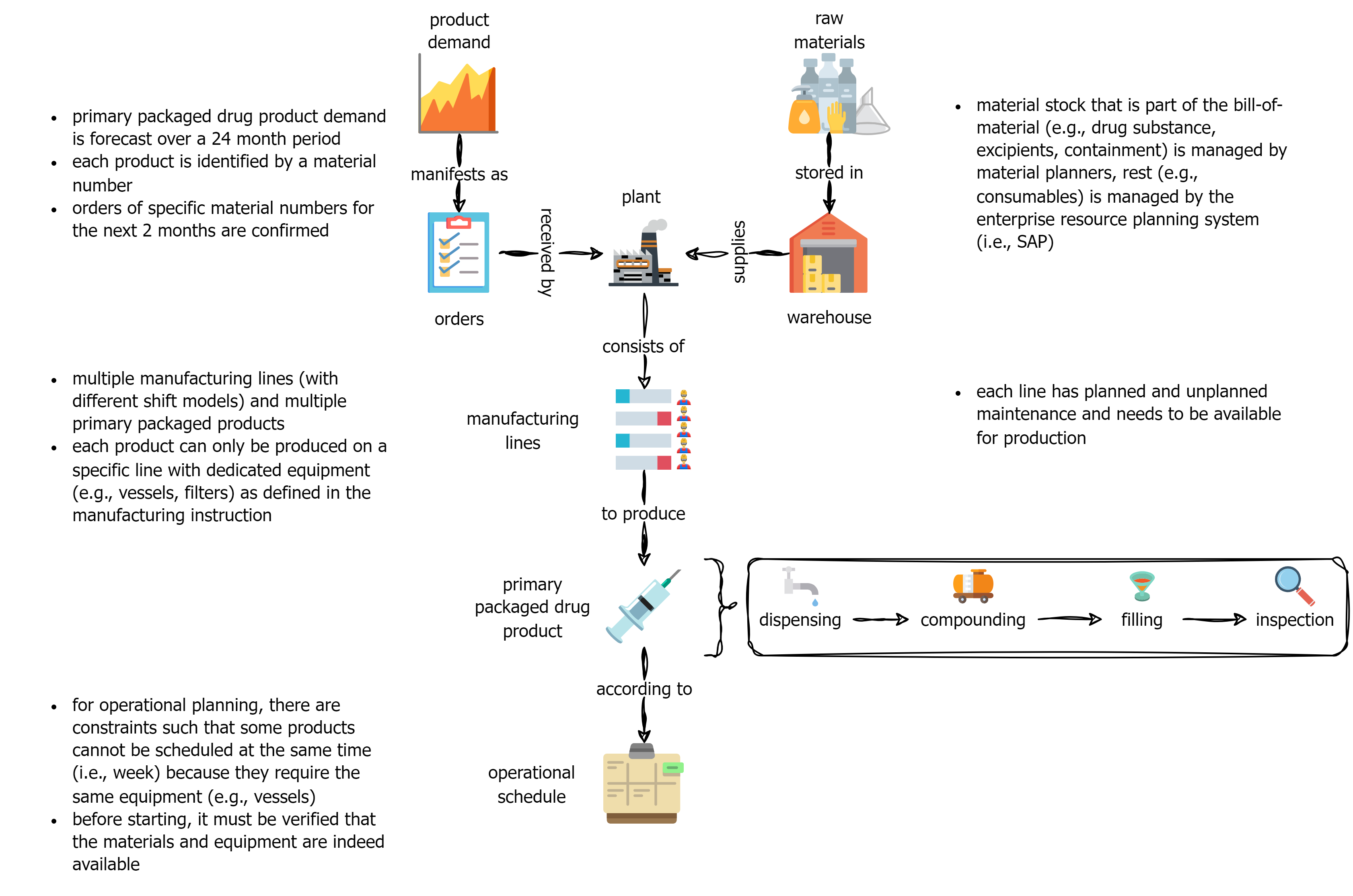
1. Why data-driven operations matter
Large-molecule manufacturing is uniquely complex:
- Products are derived from microbial, mammalian, or plant cell cultures
- Processes span upstream, downstream, sterile operations, and fill-finish
- Batch records can exceed thousands of parameters
- Investigations, deviations, and regulatory burden add operational overhead
Despite this complexity, the sector is rich in unused data. Sensors, alarms, historian systems, MES/EBR entries, ERP data, quality records, deviations, and SOPs represent a dense landscape of process knowledge (Fig. 2).
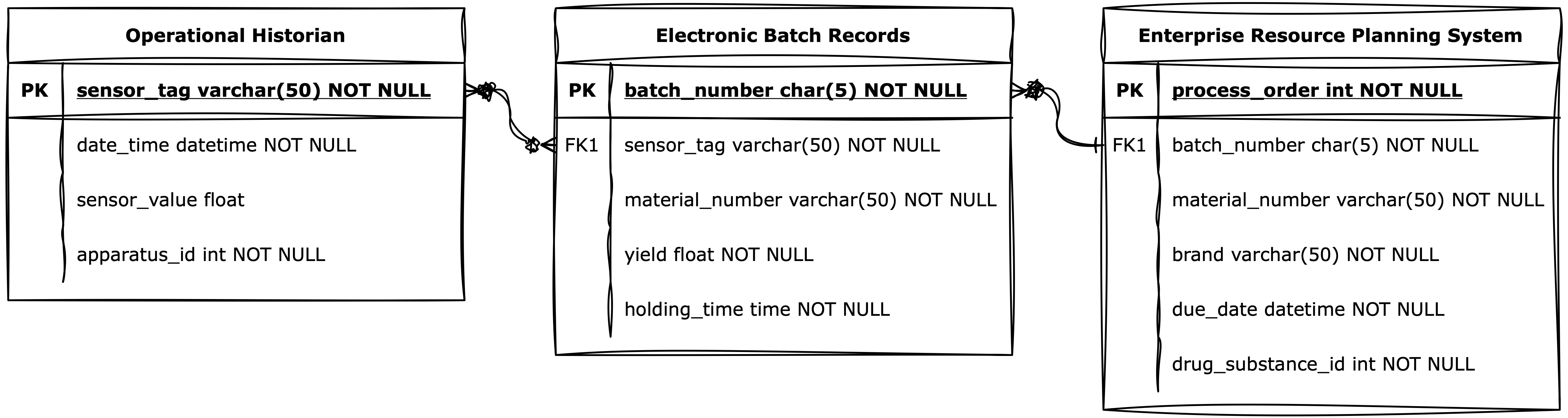
But this knowledge is rarely integrated.
The opportunity is not merely automation or dashboards. The opportunity is organizational intelligence: helping teams make faster, better, more consistent decisions by making process knowledge accessible, analyzable, and actionable.
2. The hidden bottleneck: manual support processes
Although physical processes may run automatically, the surrounding workflows often do not:
- Reporting requires manual data collection
- Investigations depend on local, tacit knowledge
- Planning cycles rely on offline spreadsheets
- Troubleshooting is reactive instead of predictive
- Documentation is hard to navigate and even harder to interpret
These activities consume disproportionate time and attention. They slow down batch release, reduce productivity, and distract teams from continuous improvement.
Digital leaders increasingly recognize that true productivity lies not in automating equipment, but in automating and augmenting the work around the equipment.
3. A model for data-driven manufacturing operations
A practical transformation model includes four pillars:
Pillar A — Accessible Knowledge
Make operational knowledge searchable and retrievable. This includes:
- SOPs
- Manufacturing instructions
- Deviation and investigation reports
- Validation documents
- Regulatory guidance
- Process descriptions
Modern retrieval-augmented generation (RAG) systems can lift these documents out of static repositories and into conversational, context-aware workflows (Fig. 3).
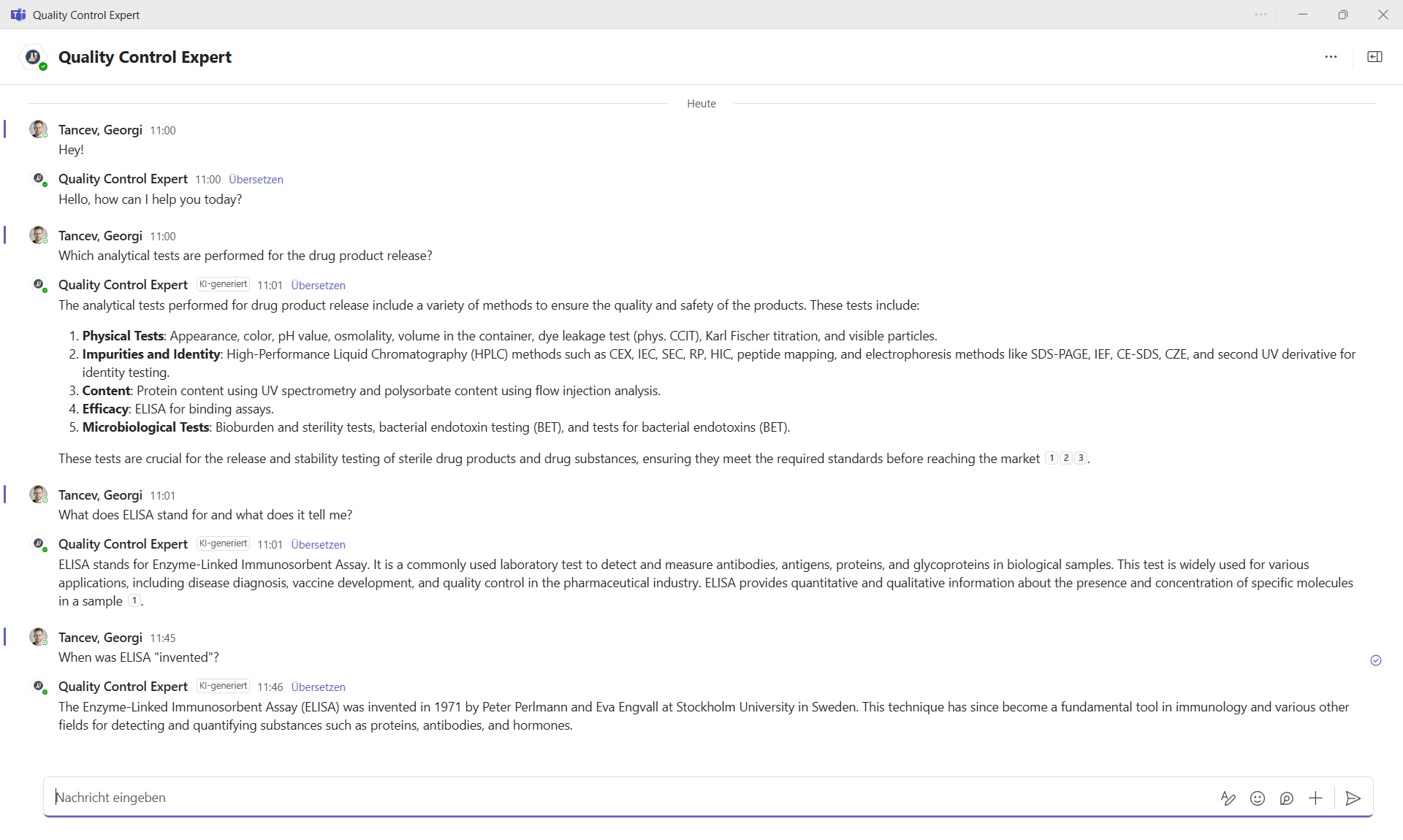
Pillar B — Integrated Data
Connect process, equipment, and business data:
- Sensor & historian signals
- MES/EBR data
- Alarm logs
- ERP & supply data
- Environmental monitoring
- Laboratory systems
The goal is a unified operational representation, not another dashboard (Fig. 4).
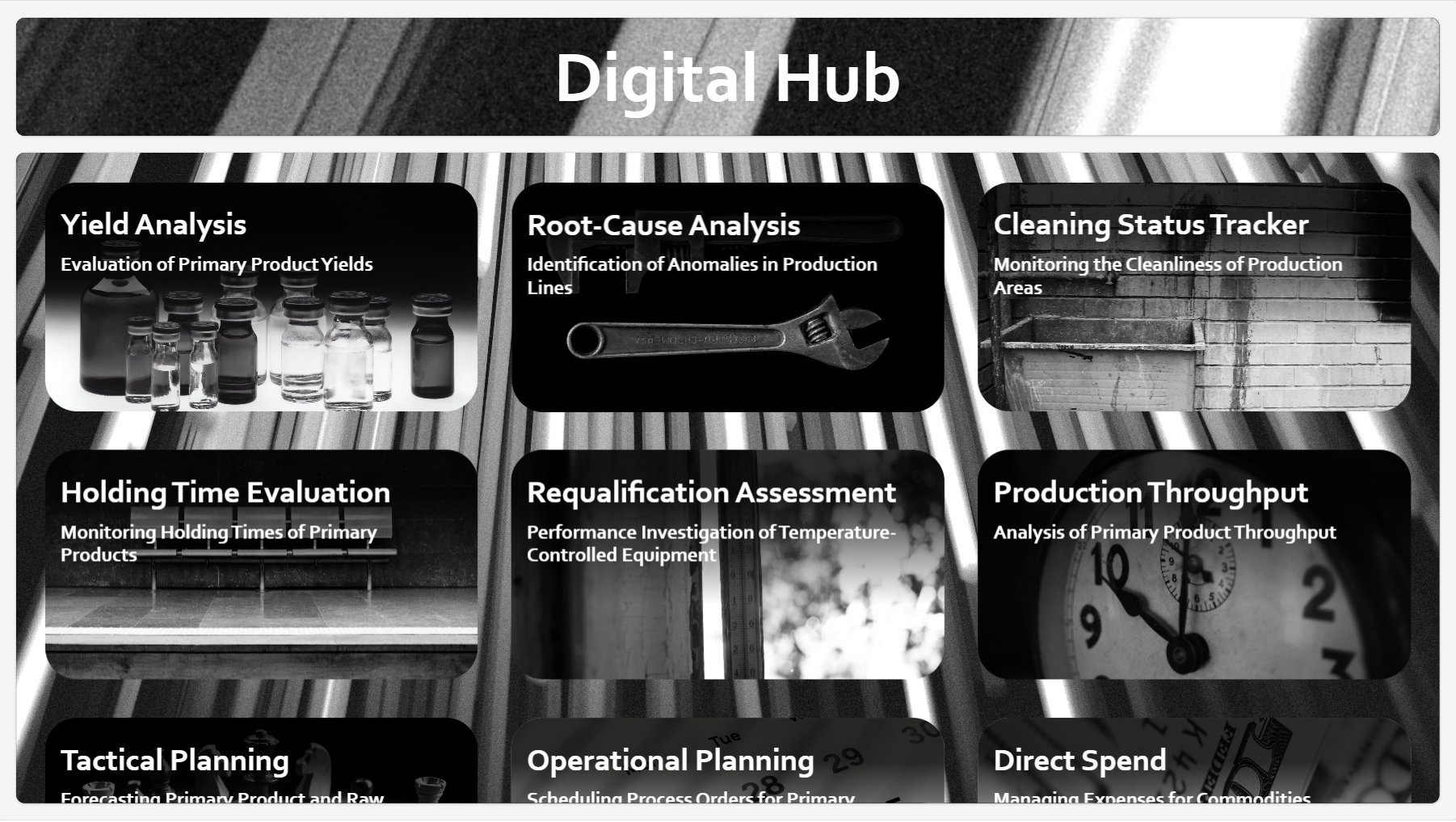
Pillar C — Decision Augmentation
Use analytics and AI to support how people work:
- Anomaly detection to spot irregularities early
- Pattern recognition in deviations or alarms
- Predictive maintenance
- Root-cause analytics
- Batch-to-batch performance comparisons
The intent is not full autonomy; it is augmented expertise.
Pillar D — Workflow Automation
Automate repetitive, structured tasks:
- Reporting
- Batch release coordination
- Data collation
- Cleaning and holding-time tracking
- Tier-meeting preparation
- KPI extraction from EBRs
Focus is on time-to-action, not simply time-to-data.
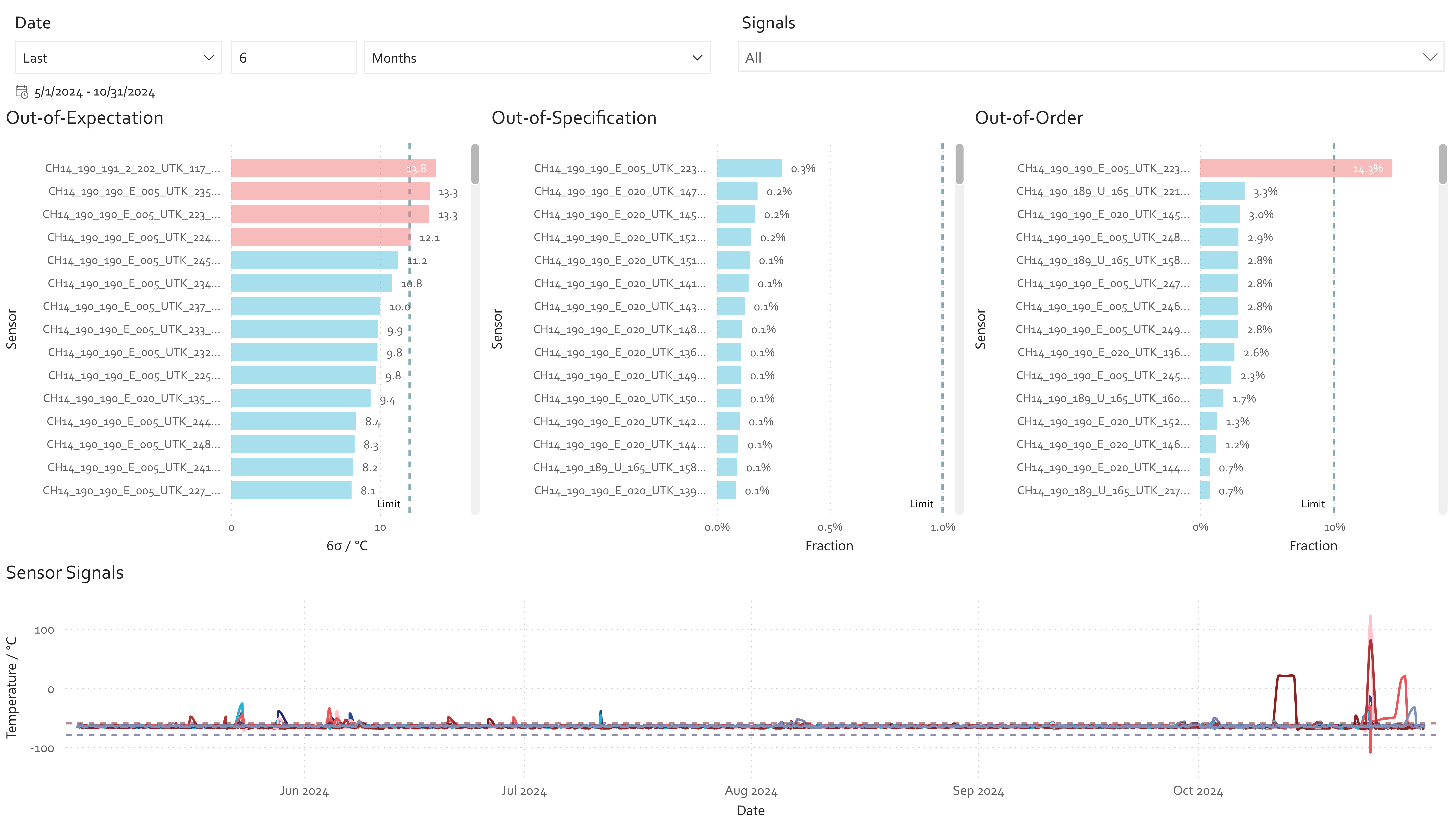
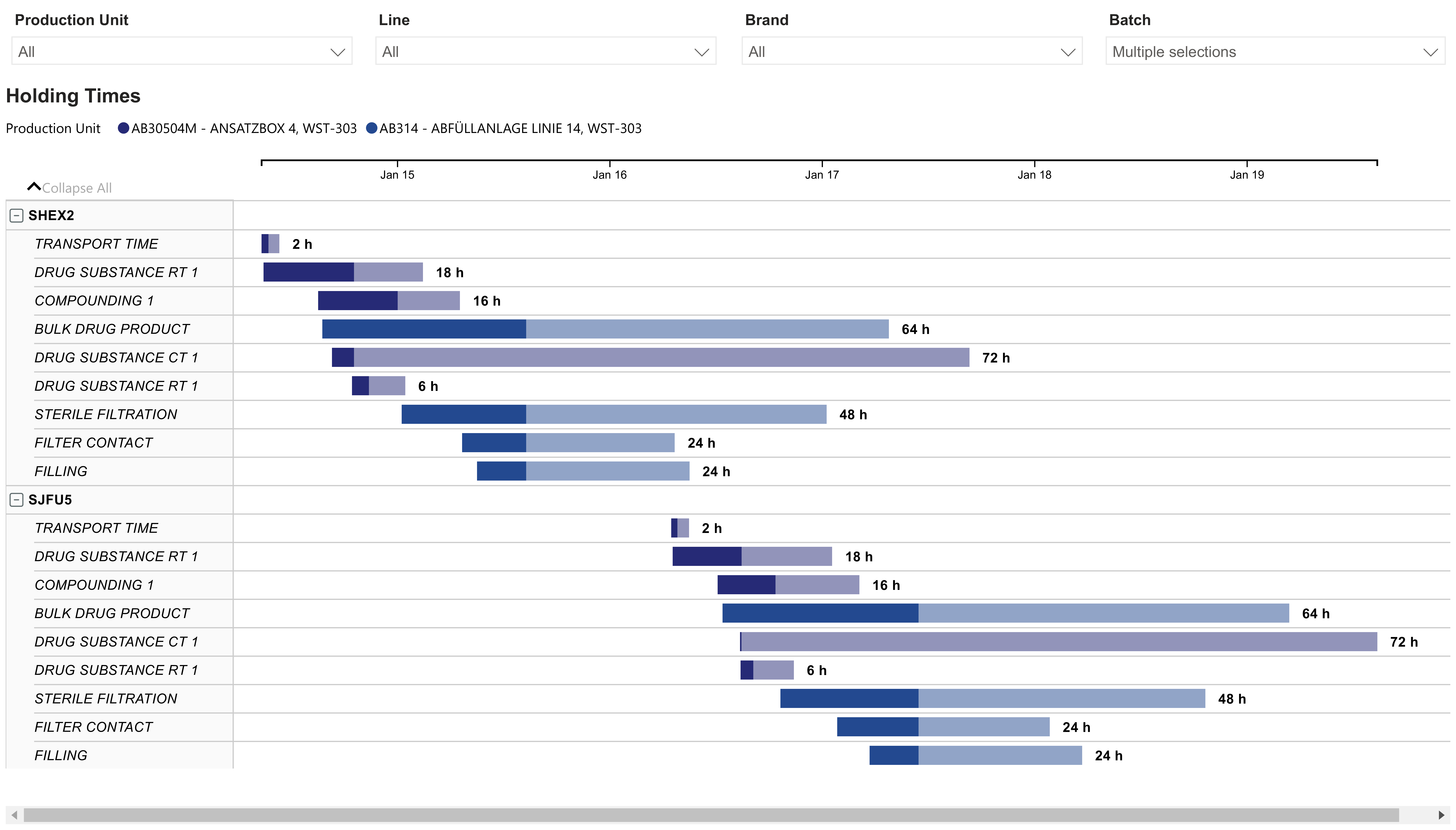
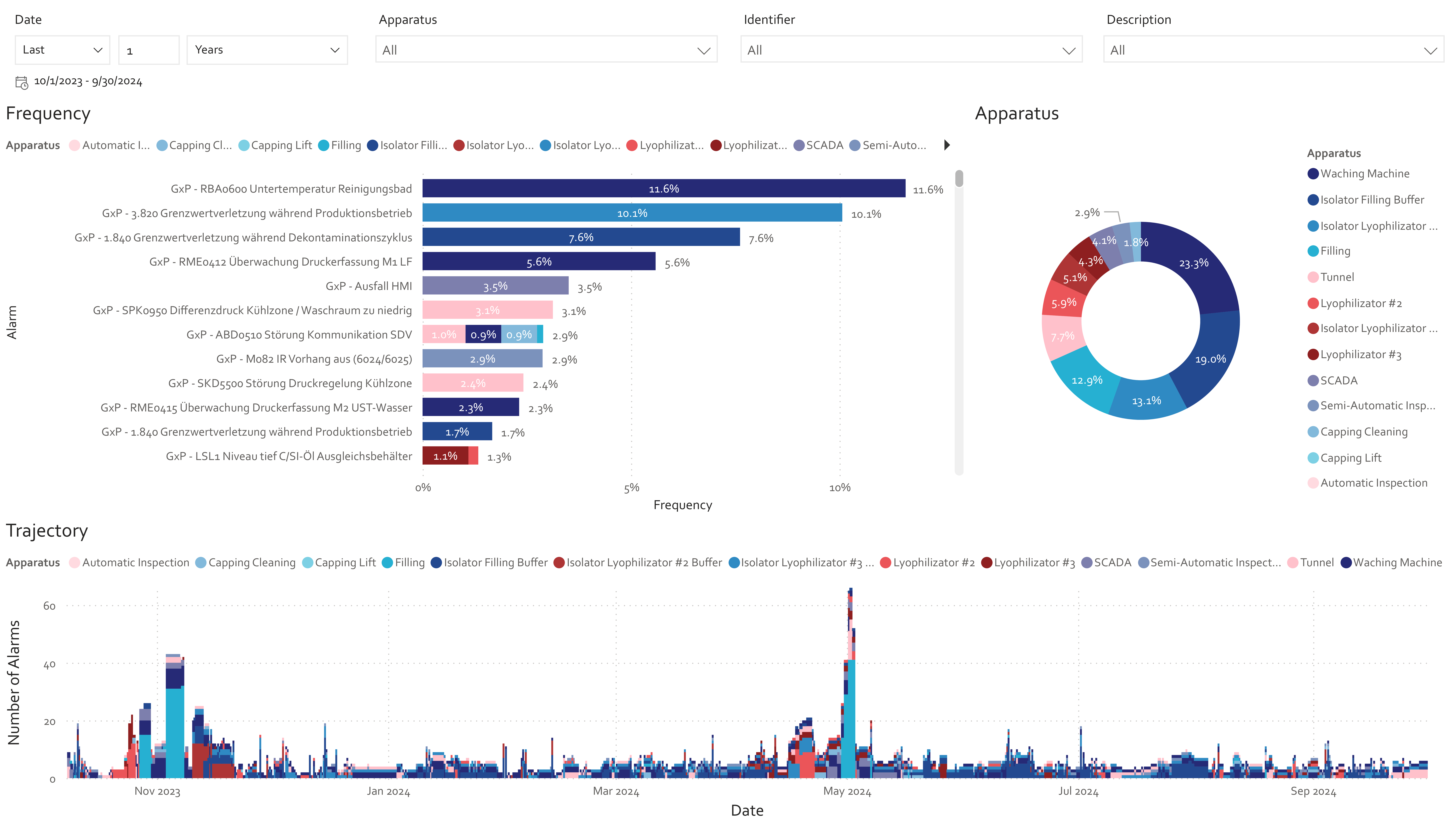
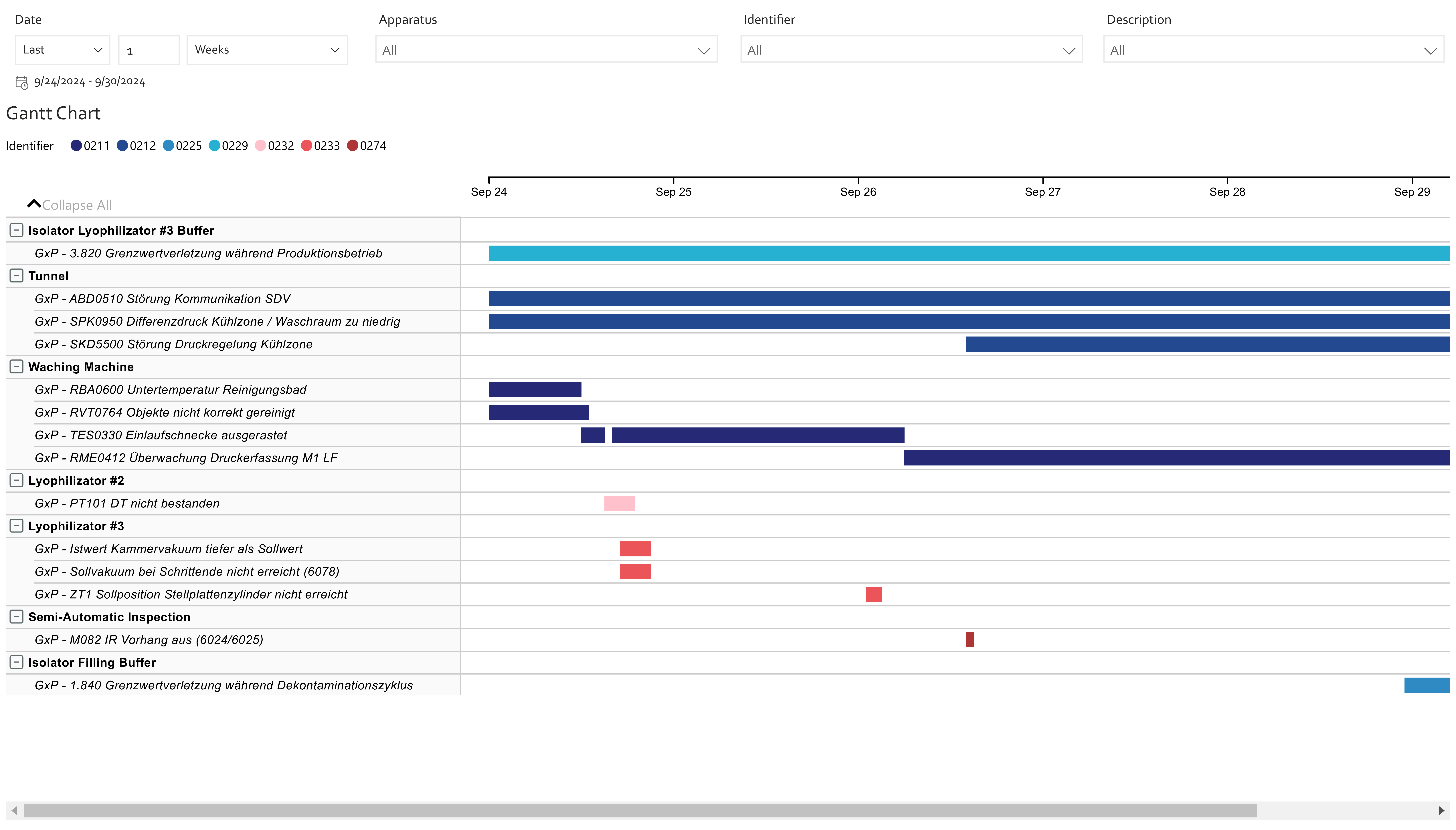
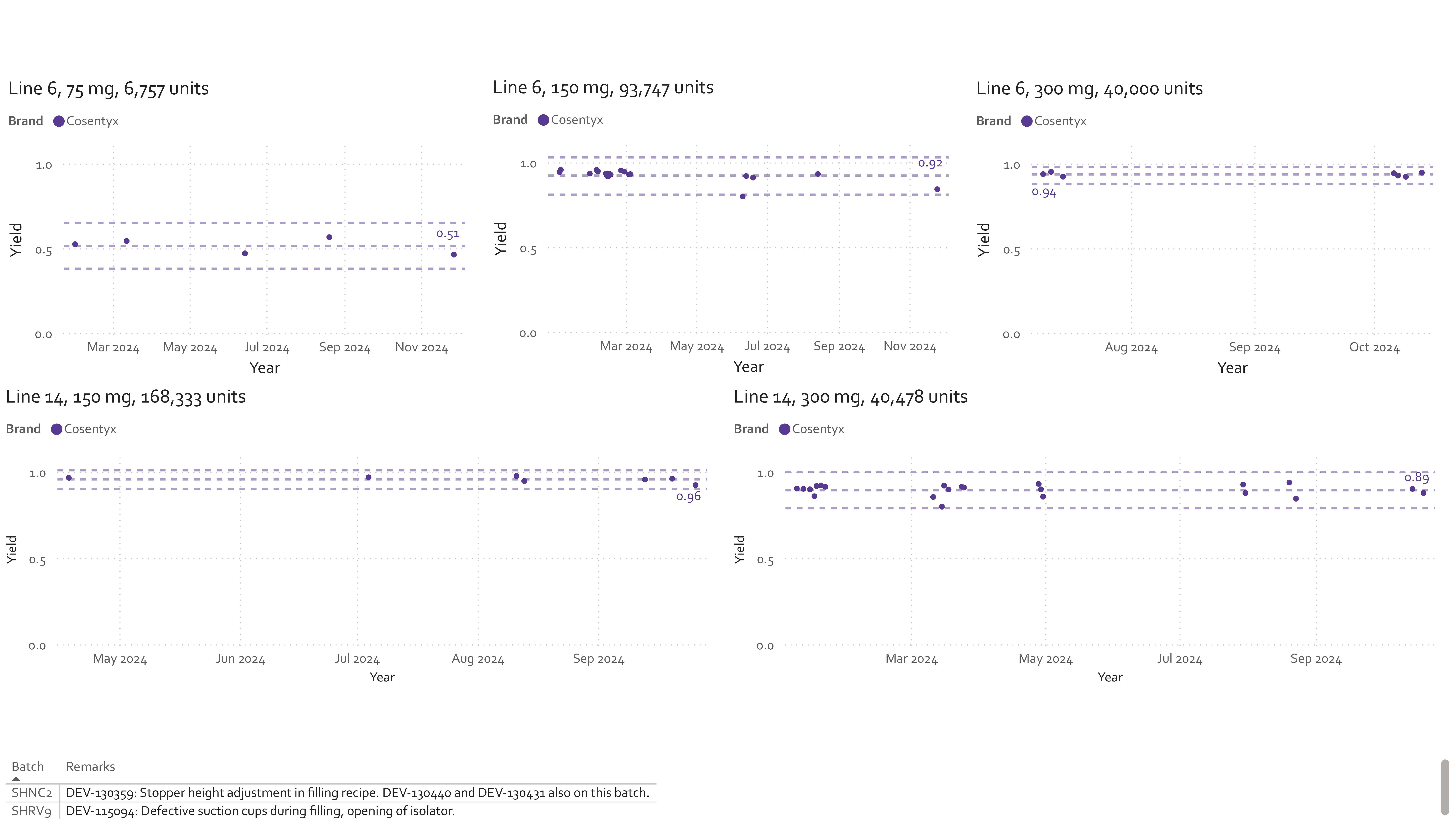
5. What this enables
A fully data-driven operating model moves teams from reactive to proactive modes:
| From | To |
|---|---|
| Searching for information | Direct answers through context-aware retrieval |
| Manual investigation | Pattern-driven diagnostics |
| KPI reporting | Automated performance insight |
| Local expertise | Institutionalized knowledge |
| “Check the systems” | “Here is what matters today” |
Digital and AI leaders in pharma increasingly align on this ambition: operational excellence through augmented decision-making.
This is not about replacing people; it is about giving teams superpowers.
6. How to get started
A practical digital-transformation journey often follows these steps:
- Identify high-friction workflows (reporting, investigations, planning)
- Map the existing data landscape (what exists, how accessible it is)
- Deploy targeted analytics on one or two use cases
- Roll out knowledge access via RAG to reduce documentation burden
- Automate supporting workflows where structure allows
- Scale horizontally across sites
Success depends on governance, trust, and adoption, not technology alone.
Closing
Data-driven manufacturing operations are not a futuristic concept — all the ingredients already exist within modern pharmaceutical sites. The challenge is orchestrating data, knowledge, and workflows into a coherent operational fabric.
With the right digital and AI strategy, manufacturing can evolve from automation of equipment to augmentation of people — turning routine operations into a resilient, intelligent, and continuously improving system.
If you want to discuss implementation, architecture, or use-case design, feel free to reach out.